NT-VRL® is a patented, data-driven terpene formulation developed and manufactured by Eybna. The formulation contains terpenes with a high indication for their anti-inflammatory & antiviral properties, in optimized ratios.
The formulation is based on Eybna’s proprietary phytochemical database, utilizing Eybna’s advanced data-mining and formulation design methodologies, to develop an effective and safe formulation. In order to evaluate NT-VRL® efficacy, the formulation was tested in two validated biological in-vitro models.
NT-VRL® exhibited inhibition of pro-inflammatory cytokines and antiviral activities, and even more profoundly when combined with CBD.
Eybna’s Data-Driven Approach
The hemp plant consists of a wide variety of phytochemicals working together to create its unique therapeutic qualities.
Eybna collects and studies the different phytochemicals found in botanical compounds and in other essential oils to learn their chemical, physical, and biological properties to utilize them for human-targeted consumption and different applications.
Eybna’s phytochemical database aggregates a wide variety of in-vitro models as well as in-vivo studies together with modern computer-aided in-silico techniques.
Eybna Data-Driven functional formulations are designed for efficacy, safety, and synergetic qualities, bringing scientific evidence to wellness products.
The Studies
NT-VRL’s In Vitro Anti-Inflammatory Evaluation
The Cytokine Storm Assay is a well-established preclinical in-vitro assay using cells obtained from healthy donors making this model an ex-vivo model.
The primary goal is to assess the effect of the test compounds on pro-inflammatory cytokine secretion inhibition levels in the human immune system resembling the cytokine storm.
NT-VRL’s In Vitro Anti-Viral Evaluation Against Coronavirus 229E
The antiviral assay is focused on assessing the antiviral activities of several test items including NT-VRL, CBD, Glycerrhizin and Pyrazofurin against Human coronavirus 229E (HCoV-229E) in human normal lung fibroblasts in vitro. The HCoV-229E virus is one of the first coronavirus strains being described and is linked to common cold symptoms.
The study’s primary goal was to assess the potential antiviral activity of the test items and to understand the mode of the antiviral action of these test items.
Our Research Partners
The two assays were carried out in partnership with CannaSoul Analytics and Pharmaseed.

The Study Results
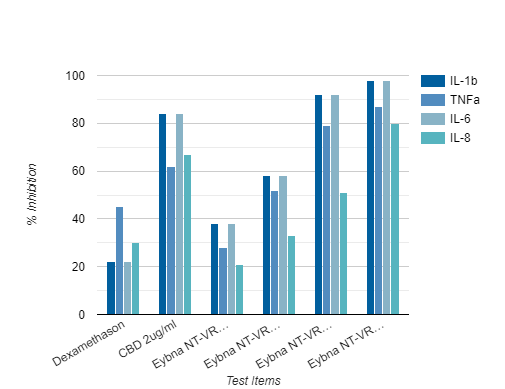
Anti-Inflammatory Evaluation
Results for all four cytokines show that NT-VRL™ formulation had a dose-dependent inhibitory effect on the secretion of pro-inflammatory cytokines, without compromising cell viability
• NT-VRL® formulation showed better inhibition than 1μg/ml dexamethasone
• Addition of CBD (2 μg/ml) together with the NT-VRL® formulation exerted the highest inhibition of cytokine secretion.
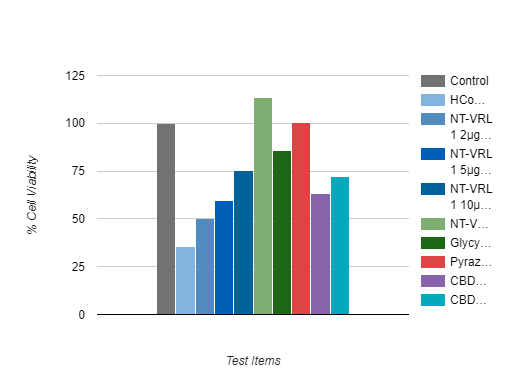
Anti-Viral Evaluation
The tested formulation exhibited an antiviral effect when it was pre-incubated with the host cells prior to virus infection. Pre-incubation of the cells with NT-VRL® prior to virus inoculation rescued the cells and increased the level of cell viability.
The combination of NT-VRL® with CBD potentiated the antiviral effect better than the positive controls pyrazofurin and glycyrrhizin.
In this study, we report the antiviral activity of the NT-VRL® terpene formulation and show that that activity was enhanced when it was applied together with CBD, suggesting either a synergetic or additive effect between the terpene formulation and CBD.
The results may indicate the NT-VRL’s antiviral effect is based on the prevention of viral attachment and/or entry.
Conclusions
The potential of phytochemicals, such as terpenes, for use as potent antiviral and anti-inflammatory agents has received considerable attention, especially because these substances are botanically-derived, naturally abundant with relatively low toxicity.
In these above studies, we report for the first time, the anti inflammatory and antiviral activity of NT-VRL® and show that these activities were enhanced when NT-VRL® was applied together with CBD, suggesting either a synergetic or additive effect. With a clinical trial coming up soon, Eybna’s plan is to prove NT-VRL’s medicinal value on patients.
Certifications
Product Applications
-
Patent Protection
-
Food Grade
-
Only Natural Ingredients
-
Solvent Free
-
Botanical Derived
-
OU Kosher
-
Non GMO
-
 Native to the Plant
Native to the Plant
For more information and business opportunities contact us